- 联系我们
- 语言 (Select Language)
- English

RESEARCH
TCM Research:
ACMS INSTITUTIONAL REVIEW BOARD (IRB)
Chinese version is currently not available
About Us
The Academy of Chinese Medicine, Singapore – Institutional Review Board (ACMS-IRB) is established under ACMS Council. This Board (also referred to as the Research Ethics Committee in some countries) is an independent Traditional Chinese Medicine (TCM) ethics review committee which reviews, approves and monitors the ethical aspects of TCM research projects conducted by a Traditional Chinese Medicine Practitioner Board (TCMPB) registered Traditional Chinese Medicine Practitioner (TCMP). Our main objective is to protect the rights and welfare of potential research subjects and also promote high ethical standards in TCM research.
At present, the ACMS-IRB would only be accepting applications for non-interventional research projects.
Overview
An overview of the initial application submission and review process is as follows:
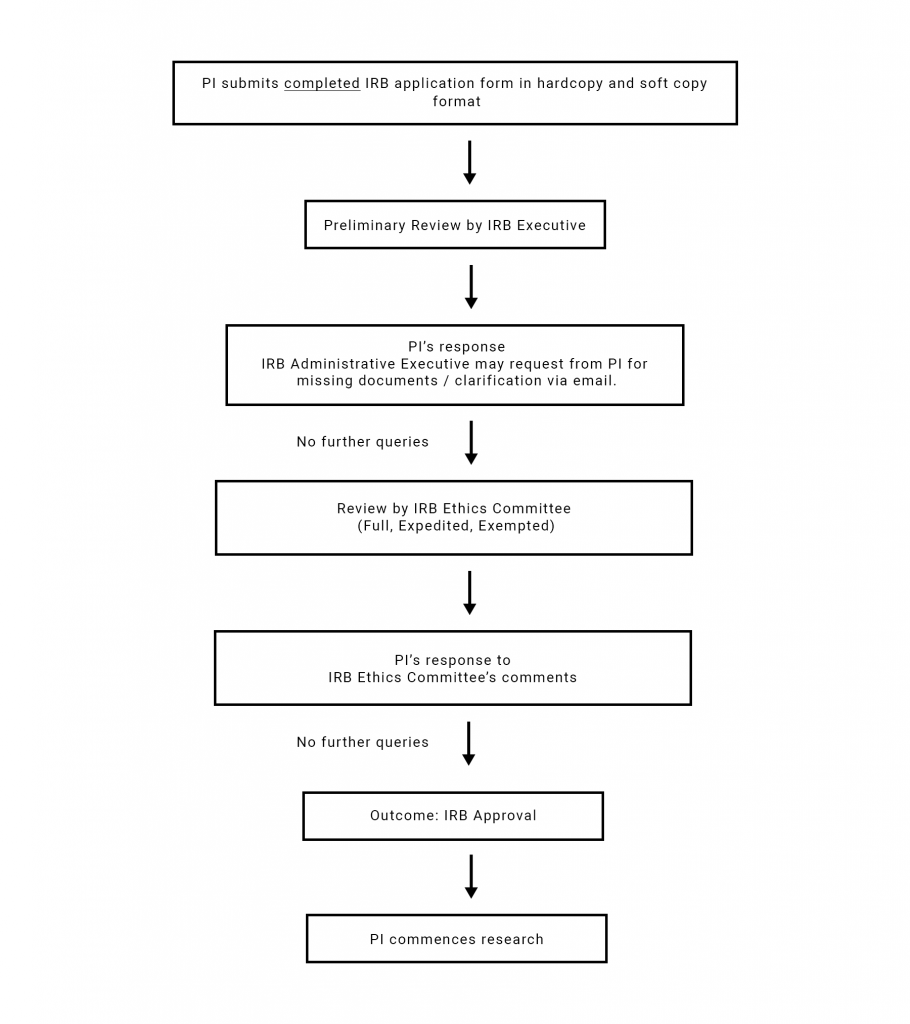
ACMS-IRB shall provide researcher(s) with an initial outcome of its review of the proposed research project 2 weeks after the IRB review.
For simple, straightforward Protocol Amendments (i.e. changes in Principal Investigator, Co-Investigator(s) and/or contact information), ACMS-IRB need to be informed within 7 working days. Otherwise, it would follow the above timeline for the new application.
The PI is to submit written summaries of the progress of the research to ACMS-IRB every quarterly.
All unexpected serious adverse events related to the research would need to be reported immediately to ACMS-IRB.
Forms Submission
Principal Investigators should use the document templates available (If applicable) for download below:
- ACMS-IRB Application Form
- Participant Information Sheet and Consent Form (Research Study)
- Participant Information Sheet and Consent Form (Video and Photography)
- Lay Summary Guideline
- Protocol (Research Study)
- Certification of Translation
- ACMS-IRB Child/Participant Assent Form
- Research involving Pregnant Women, Foetuses and Neonates
- Research involving Prisoners
- Guidelines to Advertisements for Recruitment of Research Participants
Resources
Applications are accepted throughout the year. Principal Investigators (PI) are expected to follow the submission guidelines strictly in the IRB Application form.
Softcopies of completed applications and requisite supporting documents of the following requirements should be submitted by email to research@academycms.org.
For ease of reference, please use the email subject heading “ACMS-IRB Application – [Simplified Study Title]’ (e.g. “ACMS-IRB Application – Effect of XX”) and addressed email to secretariat of ACMS-IRB.
| Document | Format |
| ACMS IRB Application Form | Word AND PDF (signed and scanned in full colour) |
| |
Other Supporting Documents If Applicable:
*Please submit these documents in additional languages, e.g. Mandarin, Malay and Tamil (if applicable). Do note that PIs are responsible for the accuracy of the translations. | Word |
The original signed hard copy of application form and supporting documents (CVs of PI, Co-I and Collaborators) should be mailed to:
Secretariat of ACMS-IRB
Academy of Chinese Medicine, Singapore
705 Serangoon Road Singapore 328127
For changes in the protocol, ACMS-IRB should be informed within 7 working days.
Will be updated soon
Fees & Payment
| Types of Review | ACMS* | Non-ACMS | |
| Initial Application | Clinical Trial which includes intervention of TCM herbs/ acupuncture/ manipulation [*Currently Not Available for Review] | 200 | 400 |
| Questionnaire/Survey/Interview | 100 | 200 | |
| Medical Records Review | 50 | 100 | |
| Subsequent Amendments | 25 | 50 | |
| Subsequent Site Amendments | 50 | 100 | |
*This is applicable to studies at host institution related to Individual ACMS members or initiated by industry or commercial entities. ACMS initiated research projects would not need a review fee.
Payment
Payment must be made payable to Academy of Chinese Medicine, Singapore by Cheque or Bank Transfer.
The cheque with cover sheet:
Must be submitted and mail to us at
Secretariat of ACMS-IRB
Academy of Chinese Medicine, Singapore
705 Serangoon Road, Singapore 328127
For Bank Transfer with cover sheet:
To provide proof of payment slip/screen shot of payment and email us at research@academycms.org.
Download Cover sheet
Contact Us
For queries, you may refer to the Frequently Answered Questions (will be updated soon) or contact the ACMS-IRB Executive at research@academycms.org.